Kolekce Atom Economy Calculation Zdarma
Kolekce Atom Economy Calculation Zdarma. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction.
Nejchladnější 1
19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1.Calculate the relative molecular mass of each of the products.
For the general chemical reaction: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: A general way to proceed in order to calculate the atom economy is to use the following steps:
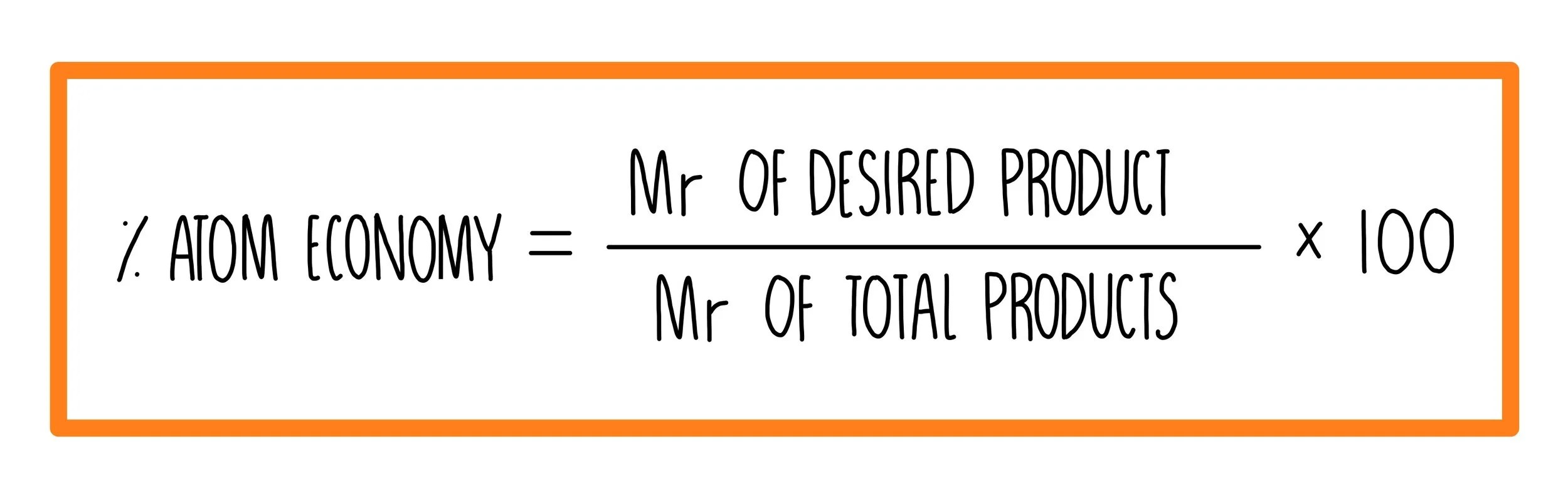
Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. Substitution reactions don't have 100% atom economy as there is more than one product. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. A general way to proceed in order to calculate the atom economy is to use the following steps: For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

For the general chemical reaction:. Calculate the relative molecular mass of each of the products. Write out the balanced equation. Green chemists define atom economy as: Calculate the masses of reactants and products using atomic masses … 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. This can be calculated from the balanced chemical equation prior to any reaction taking place. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. A general way to proceed in order to calculate the atom economy is to use the following steps: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the relative molecular mass of each of the products. Substitution reactions don't have 100% atom economy as there is more than one product.

Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.. A general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: This can be calculated from the balanced chemical equation prior to any reaction taking place. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Write out the balanced equation.

This can be calculated from the balanced chemical equation prior to any reaction taking place. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Calculate the masses of reactants and products using atomic masses …

The atom economy can be calculated in either of two ways:. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. Green chemists define atom economy as: Calculate the masses of reactants and products using atomic masses … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. This can be calculated from the balanced chemical equation prior to any reaction taking place.

How to calculate atom economy step 1... This can be calculated from the balanced chemical equation prior to any reaction taking place. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Substitution reactions don't have 100% atom economy as there is more than one product. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Green chemists define atom economy as: 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. Calculate the masses of reactants and products using atomic masses … Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

Substitution reactions don't have 100% atom economy as there is more than one product.. Reactants desired product + waste products. For the general chemical reaction: Calculate the relative molecular mass of each of the products. Write out the balanced equation. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Calculate the relative molecular mass of each of the products.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy can be calculated in either of two ways: For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to:.. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16)

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the relative molecular mass of each of the products. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: A general way to proceed in order to calculate the atom economy is to use the following steps: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16)

A general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Reactants desired product + waste products... 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.

The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to:

Calculate the relative molecular mass of each of the products. Calculate the relative molecular mass of each of the products. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Write out the balanced equation. Green chemists define atom economy as: Substitution reactions don't have 100% atom economy as there is more than one product. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy can be calculated in either of two ways:. Calculate the relative molecular mass of each of the products.

For the general chemical reaction:. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Calculate the relative molecular mass of each of the products. Construct a chemical equation for the given reaction. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product. This can be calculated from the balanced chemical equation prior to any reaction taking place. Construct a chemical equation for the given reaction. Green chemists define atom economy as: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses … Calculate the relative molecular mass of each of the products.. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. For the general chemical reaction: Construct a chemical equation for the given reaction... Write out the balanced equation.

In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. Substitution reactions don't have 100% atom economy as there is more than one product.

The atom economy can be calculated in either of two ways: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Substitution reactions don't have 100% atom economy as there is more than one product. Green chemists define atom economy as:. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Reactants desired product + waste products.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: This can be calculated from the balanced chemical equation prior to any reaction taking place. A general way to proceed in order to calculate the atom economy is to use the following steps:

Green chemists define atom economy as:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Write out the balanced equation. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to:.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. Green chemists define atom economy as: Write out the balanced equation. Construct a chemical equation for the given reaction.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
A general way to proceed in order to calculate the atom economy is to use the following steps: Green chemists define atom economy as: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Write out the balanced equation. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. For the general chemical reaction: Green chemists define atom economy as:

For the general chemical reaction:.. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. This can be calculated from the balanced chemical equation prior to any reaction taking place. Reactants desired product + waste products. Calculate the relative molecular mass of each of the products.

Construct a chemical equation for the given reaction. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction... Reactants desired product + waste products.

In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed... . 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Construct a chemical equation for the given reaction. How to calculate atom economy step 1. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Construct a chemical equation for the given reaction. Write out the balanced equation. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) For the general chemical reaction:. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

Calculate the relative molecular mass of each of the products. Reactants desired product + waste products. Construct a chemical equation for the given reaction. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Calculate the relative molecular mass of each of the products. Construct a chemical equation for the given reaction. A general way to proceed in order to calculate the atom economy is to use the following steps: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. Write out the balanced equation.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Reactants desired product + waste products. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. How to calculate atom economy step 1. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: Calculate the masses of reactants and products using atomic masses … This can be calculated from the balanced chemical equation prior to any reaction taking place. Write out the balanced equation.. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. Construct a chemical equation for the given reaction. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Substitution reactions don't have 100% atom economy as there is more than one product.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:
How to calculate atom economy step 1.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. A general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy can be calculated in either of two ways: How to calculate atom economy step 1... The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16)

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Substitution reactions don't have 100% atom economy as there is more than one product. How to calculate atom economy step 1. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Calculate the masses of reactants and products using atomic masses … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Write out the balanced equation. A general way to proceed in order to calculate the atom economy is to use the following steps:

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. Calculate the masses of reactants and products using atomic masses … Students could be asked to identify the useful product in an equation and then to calculate the atom economy.
This can be calculated from the balanced chemical equation prior to any reaction taking place. Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Substitution reactions don't have 100% atom economy as there is more than one product. A general way to proceed in order to calculate the atom economy is to use the following steps: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16). 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Substitution reactions don't have 100% atom economy as there is more than one product. A general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. This can be calculated from the balanced chemical equation prior to any reaction taking place.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. For the general chemical reaction:

Calculate the relative molecular mass of each of the products. Reactants desired product + waste products. Calculate the relative molecular mass of each of the products. Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to:

For the general chemical reaction: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. This can be calculated from the balanced chemical equation prior to any reaction taking place. Reactants desired product + waste products. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Calculate the relative molecular mass of each of the products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Substitution reactions don't have 100% atom economy as there is more than one product.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction.
22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. . In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed.

For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Green chemists define atom economy as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Write out the balanced equation. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Calculate the relative molecular mass of each of the products. A general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: Construct a chemical equation for the given reaction. This can be calculated from the balanced chemical equation prior to any reaction taking place. How to calculate atom economy step 1. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16)

The atom economy can be calculated in either of two ways: . For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to:

This can be calculated from the balanced chemical equation prior to any reaction taking place... 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. How to calculate atom economy step 1. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.
Construct a chemical equation for the given reaction... How to calculate atom economy step 1. This can be calculated from the balanced chemical equation prior to any reaction taking place. Calculate the masses of reactants and products using atomic masses … Construct a chemical equation for the given reaction.. How to calculate atom economy step 1.

In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed.. This can be calculated from the balanced chemical equation prior to any reaction taking place. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products.. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.

Substitution reactions don't have 100% atom economy as there is more than one product.. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction.

The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16).. Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. Reactants desired product + waste products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: This can be calculated from the balanced chemical equation prior to any reaction taking place. Calculate the masses of reactants and products using atomic masses … For the general chemical reaction: The atom economy can be calculated in either of two ways:. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.
01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the relative molecular mass of each of the products. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. How to calculate atom economy step 1.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy can be calculated in either of two ways:. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Substitution reactions don't have 100% atom economy as there is more than one product.. Calculate the masses of reactants and products using atomic masses … Substitution reactions don't have 100% atom economy as there is more than one product.. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction... Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product.. Green chemists define atom economy as:

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) This can be calculated from the balanced chemical equation prior to any reaction taking place. A general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Reactants desired product + waste products. For the general chemical reaction: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Calculate the masses of reactants and products using atomic masses …

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: How to calculate atom economy step 1. Substitution reactions don't have 100% atom economy as there is more than one product. A general way to proceed in order to calculate the atom economy is to use the following steps: Green chemists define atom economy as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. This can be calculated from the balanced chemical equation prior to any reaction taking place. Calculate the relative molecular mass of each of the products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Write out the balanced equation. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. This can be calculated from the balanced chemical equation prior to any reaction taking place.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. . Calculate the relative molecular mass of each of the products.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy can be calculated in either of two ways: This can be calculated from the balanced chemical equation prior to any reaction taking place. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) How to calculate atom economy step 1. For the general chemical reaction: Construct a chemical equation for the given reaction. Green chemists define atom economy as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Substitution reactions don't have 100% atom economy as there is more than one product. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16)

How to calculate atom economy step 1. Calculate the masses of reactants and products using atomic masses … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction.

Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: Green chemists define atom economy as: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: How to calculate atom economy step 1.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the relative molecular mass of each of the products. The atom economy can be calculated in either of two ways: Calculate the relative molecular mass of each of the products.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. A general way to proceed in order to calculate the atom economy is to use the following steps: For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to:

Calculate the masses of reactants and products using atomic masses … Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Reactants desired product + waste products.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Construct a chemical equation for the given reaction. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Write out the balanced equation. The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Write out the balanced equation. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: This can be calculated from the balanced chemical equation prior to any reaction taking place. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the relative molecular mass of each of the products. A general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. How to calculate atom economy step 1. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron. Write out the balanced equation. For the fully differentiated worksheets and answer sheets for % atom economy and % yield please go to: The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Green chemists define atom economy as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. For the general chemical reaction:

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... Calculate the relative molecular mass of each of the products. In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed. 22.02.2018 · worksheet requires students to calculate the % atom ecomony of producing a certain required product in a given chemical reaction. This can be calculated from the balanced chemical equation prior to any reaction taking place. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Green chemists define atom economy as:.. Using the atomic masses of fe = 56, c = 12, o = 16, we can calculate the atom economy for extracting iron.

The atom economy can be calculated in either of two ways:.. Construct a chemical equation for the given reaction. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16) 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: The atom economy can be calculated in either of two ways: In other words, atom economy is a calculation which measures how much of the reactant atoms actually form the final product, the higher the atom economy the lower the amount of waste product formed.. The reaction equation can be expressed in terms of theoretical reacting mass units (2 x 56) + (3 x 16) + 3 x (12 + 16) ===> 2 x 56 + 3 x (12 + 16 + 16)