111+ Atom With Subatomic Particles Labeled Zdarma
111+ Atom With Subatomic Particles Labeled Zdarma. Atoms with the same number of protons but different numbers of neutrons 2. A positively charged subatomic particle 3. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. These particles were electrically neutral and called neutrons.
Tady Bellwork Draw A Picture Of An Atom Label The Nucleus Protons Neutrons And Electrons 2 Give The Charges Of Each Of The 3 Subatomic Particles Ppt Download
The three main subatomic particles that form an atom are protons, neutrons, and electrons. What subatomic particles make up an atom and atoms? It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. They could explain that an atom is made up of electrons, neutrons, and protons.What subatomic particles make up an atom and atoms?
They could explain that an atom is made up of electrons, neutrons, and protons. A positively charged subatomic particle 3. What subatomic particles make up an atom and atoms? Protons, neutrons, and electrons (as seen in the helium atom below). Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The center of an atom is … It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. 15.02.2020 · particles that are smaller than the atom are called subatomic particles.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. Similarly, how many subatomic particles are there? 15.02.2020 · particles that are smaller than the atom are called subatomic particles. Total of protons and neutrons in the. Protons, neutrons, and electrons (as seen in the helium atom below). It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. Atoms with the same number of protons but different numbers of neutrons 2.

27.03.2021 · a typical atom consists of three subatomic particles: Similarly, how many subatomic particles are there? The central part of an atom containing protons and neutrons match each item with the correct statement: Protons, neutrons, and electrons (as seen in the helium atom below). 15.02.2020 · particles that are smaller than the atom are called subatomic particles.. Similarly, how many subatomic particles are there?

Total of protons and neutrons in the.. 27.03.2021 · a typical atom consists of three subatomic particles: A negatively charged subatomic particle g.

Atoms with the same number of protons but different numbers of neutrons 2... 15.02.2020 · particles that are smaller than the atom are called subatomic particles. 27.03.2021 · a typical atom consists of three subatomic particles: Similarly, how many subatomic particles are there? These particles were electrically neutral and called neutrons. They could explain that an atom is made up of electrons, neutrons, and protons. A positively charged subatomic particle 3.. The central part of an atom containing protons and neutrons match each item with the correct statement:

The center of an atom is ….. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A positively charged subatomic particle 3. What subatomic particles make up an atom and atoms? 27.03.2021 · a typical atom consists of three subatomic particles:. What subatomic particles make up an atom and atoms?

A negatively charged subatomic particle g. They could explain that an atom is made up of electrons, neutrons, and protons. Atoms with the same number of protons but different numbers of neutrons 2. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. A subatomic particle with no charge s. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. What subatomic particles make up an atom and atoms?

The center of an atom is … With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Protons, neutrons, and electrons (as seen in the helium atom below). 27.03.2021 · a typical atom consists of three subatomic particles: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A negatively charged subatomic particle g. Total of protons and neutrons in the.

27.03.2021 · a typical atom consists of three subatomic particles: .. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

There are two types of subatomic particles: Protons, neutrons, and electrons (as seen in the helium atom below). It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. There are two types of subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. What subatomic particles make up an atom and atoms? A subatomic particle with no charge s. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. A positively charged subatomic particle 3. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

What subatomic particles make up an atom and atoms? 15.02.2020 · particles that are smaller than the atom are called subatomic particles. The central part of an atom containing protons and neutrons match each item with the correct statement: These particles were electrically neutral and called neutrons. A negatively charged subatomic particle g. Atoms with the same number of protons but different numbers of neutrons 2. 27.03.2021 · a typical atom consists of three subatomic particles: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Similarly, how many subatomic particles are there? There are two types of subatomic particles: With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. A negatively charged subatomic particle g.

The center of an atom is …. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. The center of an atom is …

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Atoms with the same number of protons but different numbers of neutrons 2. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of an atom is … Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. 15.02.2020 · particles that are smaller than the atom are called subatomic particles... These particles were electrically neutral and called neutrons.

A positively charged subatomic particle 3. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. What subatomic particles make up an atom and atoms? The center of an atom is … A subatomic particle with no charge s.

These particles were electrically neutral and called neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. These particles were electrically neutral and called neutrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. They could explain that an atom is made up of electrons, neutrons, and protons. What subatomic particles make up an atom and atoms? The three main subatomic particles that form an atom are protons, neutrons, and electrons. The central part of an atom containing protons and neutrons match each item with the correct statement: A subatomic particle with no charge s.

27.03.2021 · a typical atom consists of three subatomic particles:.. Similarly, how many subatomic particles are there? They could explain that an atom is made up of electrons, neutrons, and protons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. There are two types of subatomic particles:

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. What subatomic particles make up an atom and atoms? Total of protons and neutrons in the. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The center of an atom is … The central part of an atom containing protons and neutrons match each item with the correct statement: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of the atom is called the nucleus. A subatomic particle with no charge s. They could explain that an atom is made up of electrons, neutrons, and protons. 27.03.2021 · a typical atom consists of three subatomic particles:.. The center of the atom is called the nucleus.

A positively charged subatomic particle 3... There are two types of subatomic particles: Protons, neutrons, and electrons (as seen in the helium atom below). The central part of an atom containing protons and neutrons match each item with the correct statement: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. 15.02.2020 · particles that are smaller than the atom are called subatomic particles.

15.02.2020 · particles that are smaller than the atom are called subatomic particles... . What subatomic particles make up an atom and atoms?

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. Similarly, how many subatomic particles are there? 15.02.2020 · particles that are smaller than the atom are called subatomic particles. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

The central part of an atom containing protons and neutrons match each item with the correct statement: A positively charged subatomic particle 3. Protons, neutrons, and electrons (as seen in the helium atom below). What subatomic particles make up an atom and atoms? The central part of an atom containing protons and neutrons match each item with the correct statement: 15.02.2020 · particles that are smaller than the atom are called subatomic particles. These particles were electrically neutral and called neutrons. 27.03.2021 · a typical atom consists of three subatomic particles:. The center of an atom is …

Total of protons and neutrons in the.. The center of the atom is called the nucleus. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Total of protons and neutrons in the. Protons, neutrons, and electrons (as seen in the helium atom below)... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

The central part of an atom containing protons and neutrons match each item with the correct statement: There are two types of subatomic particles: The center of an atom is … A subatomic particle with no charge s. Total of protons and neutrons in the. Atoms with the same number of protons but different numbers of neutrons 2. 27.03.2021 · a typical atom consists of three subatomic particles: The three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. What subatomic particles make up an atom and atoms?. A subatomic particle with no charge s.

The center of the atom is called the nucleus. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Total of protons and neutrons in the. A negatively charged subatomic particle g. Total of protons and neutrons in the.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. 27.03.2021 · a typical atom consists of three subatomic particles: What subatomic particles make up an atom and atoms? The central part of an atom containing protons and neutrons match each item with the correct statement: They could explain that an atom is made up of electrons, neutrons, and protons. 15.02.2020 · particles that are smaller than the atom are called subatomic particles... Protons, neutrons, and electrons (as seen in the helium atom below).

There are two types of subatomic particles:.. . These particles were electrically neutral and called neutrons.

What subatomic particles make up an atom and atoms?. Atoms with the same number of protons but different numbers of neutrons 2. What subatomic particles make up an atom and atoms? It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A subatomic particle with no charge s. 27.03.2021 · a typical atom consists of three subatomic particles:. There are two types of subatomic particles:

15.02.2020 · particles that are smaller than the atom are called subatomic particles.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The center of the atom is called the nucleus. These particles were electrically neutral and called neutrons. A subatomic particle with no charge s. What subatomic particles make up an atom and atoms? With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. There are two types of subatomic particles: The central part of an atom containing protons and neutrons match each item with the correct statement: They could explain that an atom is made up of electrons, neutrons, and protons. A positively charged subatomic particle 3. Atoms with the same number of protons but different numbers of neutrons 2.

The center of the atom is called the nucleus.. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Protons, neutrons, and electrons (as seen in the helium atom below). The central part of an atom containing protons and neutrons match each item with the correct statement: The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The central part of an atom containing protons and neutrons match each item with the correct statement: There are two types of subatomic particles: These particles were electrically neutral and called neutrons. A subatomic particle with no charge s. 27.03.2021 · a typical atom consists of three subatomic particles: Total of protons and neutrons in the. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. A positively charged subatomic particle 3. A negatively charged subatomic particle g. The center of the atom is called the nucleus... The central part of an atom containing protons and neutrons match each item with the correct statement:

The center of the atom is called the nucleus... It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. These particles were electrically neutral and called neutrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of the atom is called the nucleus.. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

The center of the atom is called the nucleus... There are two types of subatomic particles: The three main subatomic particles that form an atom are protons, neutrons, and electrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. A subatomic particle with no charge s. 27.03.2021 · a typical atom consists of three subatomic particles: 15.02.2020 · particles that are smaller than the atom are called subatomic particles. A negatively charged subatomic particle g. What subatomic particles make up an atom and atoms? It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. What subatomic particles make up an atom and atoms?

The center of an atom is … . With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

The central part of an atom containing protons and neutrons match each item with the correct statement: Similarly, how many subatomic particles are there? Atoms with the same number of protons but different numbers of neutrons 2. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A negatively charged subatomic particle g. They could explain that an atom is made up of electrons, neutrons, and protons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. A subatomic particle with no charge s. Total of protons and neutrons in the... A negatively charged subatomic particle g.

Total of protons and neutrons in the. .. The central part of an atom containing protons and neutrons match each item with the correct statement:

15.02.2020 · particles that are smaller than the atom are called subatomic particles. What subatomic particles make up an atom and atoms?

The three main subatomic particles that form an atom are protons, neutrons, and electrons. Similarly, how many subatomic particles are there? The three main subatomic particles that form an atom are protons, neutrons, and electrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Total of protons and neutrons in the... It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

There are two types of subatomic particles: The three main subatomic particles that form an atom are protons, neutrons, and electrons. The central part of an atom containing protons and neutrons match each item with the correct statement: Protons, neutrons, and electrons (as seen in the helium atom below). Atoms with the same number of protons but different numbers of neutrons 2. The center of the atom is called the nucleus. A negatively charged subatomic particle g. Total of protons and neutrons in the. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. Similarly, how many subatomic particles are there? There are two types of subatomic particles:

27.03.2021 · a typical atom consists of three subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. A subatomic particle with no charge s. A positively charged subatomic particle 3. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Protons, neutrons, and electrons (as seen in the helium atom below). There are two types of subatomic particles: A negatively charged subatomic particle g. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. They could explain that an atom is made up of electrons, neutrons, and protons.

Atoms with the same number of protons but different numbers of neutrons 2.. There are two types of subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The central part of an atom containing protons and neutrons match each item with the correct statement: The center of an atom is … Protons, neutrons, and electrons (as seen in the helium atom below). Total of protons and neutrons in the. A subatomic particle with no charge s. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom... The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The central part of an atom containing protons and neutrons match each item with the correct statement: The center of the atom is called the nucleus. Total of protons and neutrons in the. 27.03.2021 · a typical atom consists of three subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of an atom is … These particles were electrically neutral and called neutrons... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

A negatively charged subatomic particle g. The central part of an atom containing protons and neutrons match each item with the correct statement: The center of an atom is … The center of the atom is called the nucleus. A subatomic particle with no charge s. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. 27.03.2021 · a typical atom consists of three subatomic particles: Atoms with the same number of protons but different numbers of neutrons 2. A positively charged subatomic particle 3. They could explain that an atom is made up of electrons, neutrons, and protons. A negatively charged subatomic particle g.. A positively charged subatomic particle 3.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. A positively charged subatomic particle 3. What subatomic particles make up an atom and atoms? The center of the atom is called the nucleus. They could explain that an atom is made up of electrons, neutrons, and protons. The central part of an atom containing protons and neutrons match each item with the correct statement: Similarly, how many subatomic particles are there? Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 27.03.2021 · a typical atom consists of three subatomic particles: Total of protons and neutrons in the... Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

27.03.2021 · a typical atom consists of three subatomic particles: The central part of an atom containing protons and neutrons match each item with the correct statement: The center of an atom is … Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Atoms with the same number of protons but different numbers of neutrons 2. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A subatomic particle with no charge s. Protons, neutrons, and electrons (as seen in the helium atom below). These particles were electrically neutral and called neutrons. Total of protons and neutrons in the. Similarly, how many subatomic particles are there?. A positively charged subatomic particle 3.

What subatomic particles make up an atom and atoms?.. What subatomic particles make up an atom and atoms? The central part of an atom containing protons and neutrons match each item with the correct statement: A subatomic particle with no charge s. Similarly, how many subatomic particles are there?
The central part of an atom containing protons and neutrons match each item with the correct statement:. What subatomic particles make up an atom and atoms? The center of the atom is called the nucleus. The center of an atom is …. They could explain that an atom is made up of electrons, neutrons, and protons.

These particles were electrically neutral and called neutrons. The center of the atom is called the nucleus. A negatively charged subatomic particle g. A subatomic particle with no charge s. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons. There are two types of subatomic particles:. The center of an atom is …

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A positively charged subatomic particle 3. Total of protons and neutrons in the.

They could explain that an atom is made up of electrons, neutrons, and protons. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. What subatomic particles make up an atom and atoms? These particles were electrically neutral and called neutrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons, neutrons, and electrons (as seen in the helium atom below). The central part of an atom containing protons and neutrons match each item with the correct statement: Total of protons and neutrons in the.

The center of an atom is … A subatomic particle with no charge s. What subatomic particles make up an atom and atoms? Protons, neutrons, and electrons (as seen in the helium atom below). They could explain that an atom is made up of electrons, neutrons, and protons. Total of protons and neutrons in the. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms... Atoms with the same number of protons but different numbers of neutrons 2.

They could explain that an atom is made up of electrons, neutrons, and protons. A subatomic particle with no charge s. A negatively charged subatomic particle g. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. Protons, neutrons, and electrons (as seen in the helium atom below).

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom... . The three main subatomic particles that form an atom are protons, neutrons, and electrons.

These particles were electrically neutral and called neutrons. A subatomic particle with no charge s. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. A negatively charged subatomic particle g. Similarly, how many subatomic particles are there? Total of protons and neutrons in the.

The center of the atom is called the nucleus. A negatively charged subatomic particle g. Total of protons and neutrons in the. Protons, neutrons, and electrons (as seen in the helium atom below). The center of the atom is called the nucleus... 15.02.2020 · particles that are smaller than the atom are called subatomic particles.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. . With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

27.03.2021 · a typical atom consists of three subatomic particles:.. Atoms with the same number of protons but different numbers of neutrons 2. These particles were electrically neutral and called neutrons. A subatomic particle with no charge s. 27.03.2021 · a typical atom consists of three subatomic particles: The central part of an atom containing protons and neutrons match each item with the correct statement: What subatomic particles make up an atom and atoms? Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons... There are two types of subatomic particles:

The three main subatomic particles that form an atom are protons, neutrons, and electrons. The central part of an atom containing protons and neutrons match each item with the correct statement: These particles were electrically neutral and called neutrons.. A negatively charged subatomic particle g.

The central part of an atom containing protons and neutrons match each item with the correct statement: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Similarly, how many subatomic particles are there? What subatomic particles make up an atom and atoms?.. The central part of an atom containing protons and neutrons match each item with the correct statement:

What subatomic particles make up an atom and atoms?. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. A negatively charged subatomic particle g.

The center of an atom is … The center of an atom is … Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. A negatively charged subatomic particle g. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. A positively charged subatomic particle 3.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. What subatomic particles make up an atom and atoms? There are two types of subatomic particles: Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Similarly, how many subatomic particles are there? Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of the atom is called the nucleus. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons.. Atoms with the same number of protons but different numbers of neutrons 2.

15.02.2020 · particles that are smaller than the atom are called subatomic particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. These particles were electrically neutral and called neutrons.
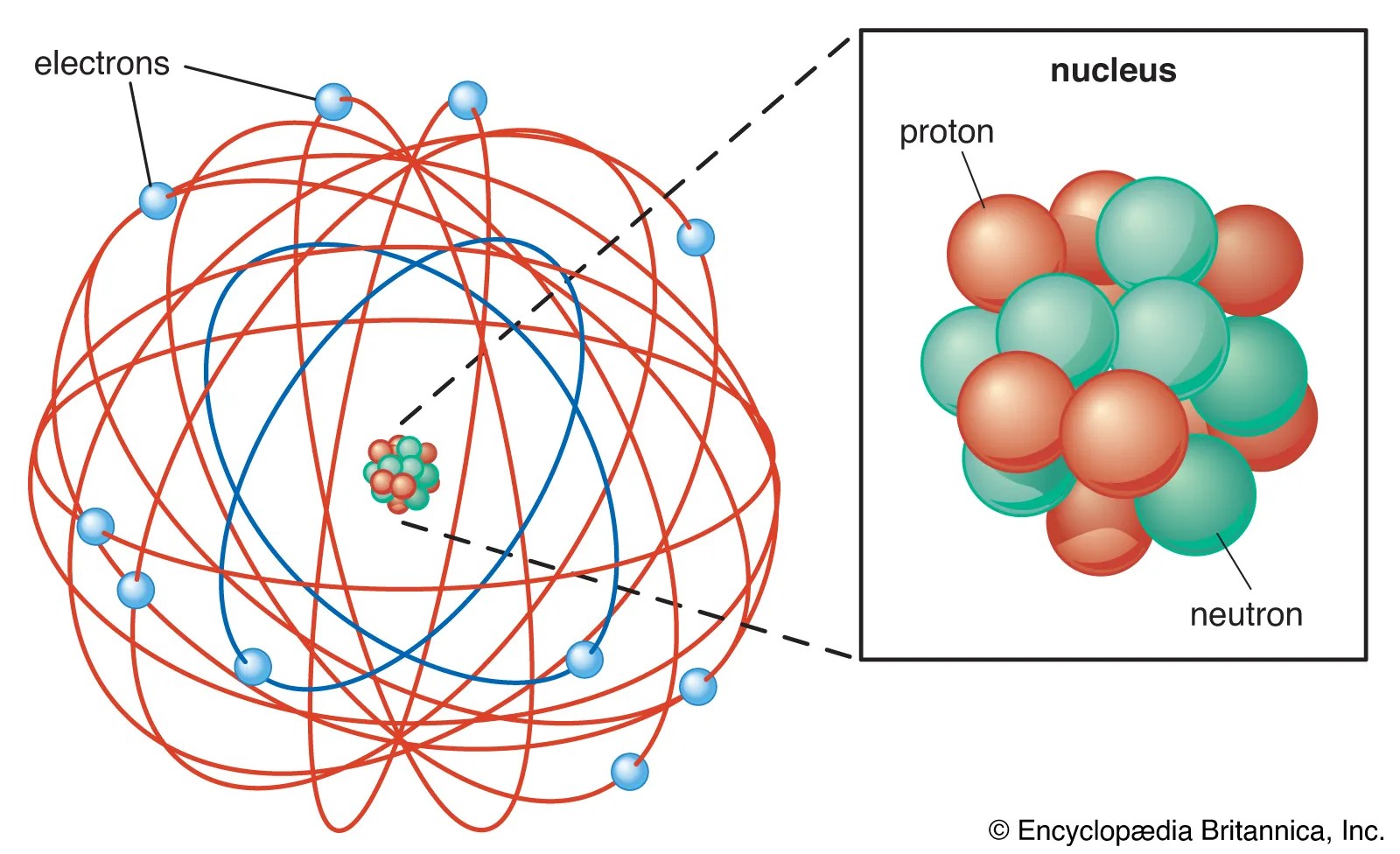
A positively charged subatomic particle 3. The center of an atom is … There are two types of subatomic particles: Similarly, how many subatomic particles are there?

Total of protons and neutrons in the... These particles were electrically neutral and called neutrons. 27.03.2021 · a typical atom consists of three subatomic particles: Total of protons and neutrons in the. They could explain that an atom is made up of electrons, neutrons, and protons. The center of an atom is … A positively charged subatomic particle 3.

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom... Protons, neutrons, and electrons (as seen in the helium atom below).. The center of the atom is called the nucleus.

15.02.2020 · particles that are smaller than the atom are called subatomic particles. They could explain that an atom is made up of electrons, neutrons, and protons. These particles were electrically neutral and called neutrons. The center of an atom is … There are two types of subatomic particles: A subatomic particle with no charge s. Similarly, how many subatomic particles are there?. They could explain that an atom is made up of electrons, neutrons, and protons.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms... These particles were electrically neutral and called neutrons... A subatomic particle with no charge s.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom... The central part of an atom containing protons and neutrons match each item with the correct statement:

27.03.2021 · a typical atom consists of three subatomic particles: With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. These particles were electrically neutral and called neutrons. A negatively charged subatomic particle g. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. 27.03.2021 · a typical atom consists of three subatomic particles:

A negatively charged subatomic particle g. There are two types of subatomic particles: Similarly, how many subatomic particles are there? It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A subatomic particle with no charge s. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. 15.02.2020 · particles that are smaller than the atom are called subatomic particles.

They could explain that an atom is made up of electrons, neutrons, and protons. Atoms with the same number of protons but different numbers of neutrons 2. A positively charged subatomic particle 3. The center of an atom is … There are two types of subatomic particles: The central part of an atom containing protons and neutrons match each item with the correct statement: Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. A negatively charged subatomic particle g.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. These particles were electrically neutral and called neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The central part of an atom containing protons and neutrons match each item with the correct statement: A subatomic particle with no charge s. A positively charged subatomic particle 3.. The center of an atom is …

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Atoms with the same number of protons but different numbers of neutrons 2. A subatomic particle with no charge s. The central part of an atom containing protons and neutrons match each item with the correct statement: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A negatively charged subatomic particle g. Similarly, how many subatomic particles are there? What subatomic particles make up an atom and atoms? 27.03.2021 · a typical atom consists of three subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
There are two types of subatomic particles:. There are two types of subatomic particles: 15.02.2020 · particles that are smaller than the atom are called subatomic particles. The central part of an atom containing protons and neutrons match each item with the correct statement: 27.03.2021 · a typical atom consists of three subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. A subatomic particle with no charge s. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. Similarly, how many subatomic particles are there?

A negatively charged subatomic particle g... With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The center of an atom is … Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. These particles were electrically neutral and called neutrons. They could explain that an atom is made up of electrons, neutrons, and protons. 15.02.2020 · particles that are smaller than the atom are called subatomic particles. 27.03.2021 · a typical atom consists of three subatomic particles: The center of the atom is called the nucleus. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of the atom is called the nucleus.

Total of protons and neutrons in the. There are two types of subatomic particles: Protons, neutrons, and electrons (as seen in the helium atom below). A subatomic particle with no charge s. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A negatively charged subatomic particle g. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Atoms with the same number of protons but different numbers of neutrons 2. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. They could explain that an atom is made up of electrons, neutrons, and protons. A negatively charged subatomic particle g. A subatomic particle with no charge s. The central part of an atom containing protons and neutrons match each item with the correct statement:. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The center of the atom is called the nucleus. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Atoms with the same number of protons but different numbers of neutrons 2. The center of an atom is … The three main subatomic particles that form an atom are protons, neutrons, and electrons. What subatomic particles make up an atom and atoms? They could explain that an atom is made up of electrons, neutrons, and protons. Total of protons and neutrons in the.. They could explain that an atom is made up of electrons, neutrons, and protons.

A negatively charged subatomic particle g.. A subatomic particle with no charge s. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. What subatomic particles make up an atom and atoms? Similarly, how many subatomic particles are there? Protons, neutrons, and electrons (as seen in the helium atom below). Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Total of protons and neutrons in the.

A subatomic particle with no charge s. A negatively charged subatomic particle g... A negatively charged subatomic particle g.

Atoms with the same number of protons but different numbers of neutrons 2. A positively charged subatomic particle 3. The center of an atom is … Total of protons and neutrons in the. A negatively charged subatomic particle g. 27.03.2021 · a typical atom consists of three subatomic particles: 15.02.2020 · particles that are smaller than the atom are called subatomic particles. These particles were electrically neutral and called neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Similarly, how many subatomic particles are there?.. A positively charged subatomic particle 3. What subatomic particles make up an atom and atoms? 27.03.2021 · a typical atom consists of three subatomic particles: Similarly, how many subatomic particles are there? There are two types of subatomic particles: With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. A subatomic particle with no charge s.

Total of protons and neutrons in the... Similarly, how many subatomic particles are there? The three main subatomic particles that form an atom are protons, neutrons, and electrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. They could explain that an atom is made up of electrons, neutrons, and protons. These particles were electrically neutral and called neutrons.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Similarly, how many subatomic particles are there? It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. There are two types of subatomic particles: The three main subatomic particles that form an atom are protons, neutrons, and electrons. A positively charged subatomic particle 3. A subatomic particle with no charge s. A negatively charged subatomic particle g. 27.03.2021 · a typical atom consists of three subatomic particles:. They could explain that an atom is made up of electrons, neutrons, and protons.

The central part of an atom containing protons and neutrons match each item with the correct statement: 15.02.2020 · particles that are smaller than the atom are called subatomic particles. A positively charged subatomic particle 3. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

They could explain that an atom is made up of electrons, neutrons, and protons... The center of an atom is … The central part of an atom containing protons and neutrons match each item with the correct statement: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons, neutrons, and electrons (as seen in the helium atom below). With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. A subatomic particle with no charge s. Total of protons and neutrons in the. Similarly, how many subatomic particles are there? A positively charged subatomic particle 3. There are two types of subatomic particles: There are two types of subatomic particles: